This Vaccine Alternative Could Be A Big Winner Invivyd (ivvd – Nasd)
In the US in 2024, Covid vaccine uptake was 23% among adults and 14% among children. That works out to 70-80 million people that received a Covid vaccine…YEARS after the pandemic.
INVIVYD (IVVD-NASD) is in Phase 3 trial for a new drug that would be a vaccine ALTERNATIVE–which in this political environment is a big plus.
This story outlines the history of IVVD, its drug and how it could be even better than vaccines. But right up top here, I want you to understand how big this $1.40 stock could be.
Lets say their Phase 3 is positive (WHO KNOWS–THERE IS STILL RISK!) If Invivyd can penetrate 10-20% of the vaccine market, which would assume penetrating most of that at-risk segment, that would be around 7.5-15 million people.
Both Moderna (MRNA – NASDAQ) and Pfizer (PFE – NYSE) shots retail at ~$200 per dose. At a recent Cantor Fitzgerald conference, IVVD Chairman of the Board Marc Elia said he thought “a turn above” the vaccine pricing would be appropriate.
At $300 per dose for 15 million people, that would be $4.5B of revenue for a single dose per year. If half of that penetrated population receives a second dose every year, that’s another $2.2B.
That puts IVVD’s drug candidate–VYD2311–well above the $1B “blockbuster” status, and we haven’t even considered the opportunity from anywhere other than the US. There is also the opportunity in long-Covid–which is 6 out of every 100 people.
So this is a story that biotech investors should be watching. It is not a risk free trade, but has asymetrical upside if successful.
QUICK FACTS
Trading Symbols: IVVD
Share Price Today: $1.40
Shares Outstanding: 209 million*
Market Capitalization: $293 million
Cash: $81 million*
Debt: $0 million
Enterprise Value: $212 million
COVID IS STILL A BIG MARKET
Today, the phrase “Covid vaccine” is a political hand grenade. Especially in the US, conservative politicians are on the offensive and stepping back any supports for Covid vaccination.
I have no interest in debating the politics here.
Speculating in stocks is about correctly detecting and jumping on a trend, not analyzing its long-run accuracy.
It’s that old saying: Do you want to be right, or do you want to make money?
The simple truth is that many trends are both wrong and profitable.
Invivyd’s monoclonal antibody should deliver the same patient experience as a vaccine. It should deliver as good or better efficacy than a vaccine, especially in patients with poor immune response. It should have better tolerability. It may even last longer.
You put that on top of the politics, and it looks a lot like Invivyd is on the right side of vaccine debate.
If the cards fall right, that would make it a big winner.
MONOCLONOL ANTIBODIES – NOT A VACCINE
The most important thing to understand about a mAb is that it can elicit the same response to a virus as a vaccine without making any changes to your body.
Vaccines work by training your body to make an antibody. With a vaccine you are exposed to something, like a dead virus, and that makes your immune system react.
In the process your immune system learns to make an antibody that can be used when the actual virus comes along.
mAbs skip the training process. The mAb is the antibody (thus the name – monoclonal antibody) that neutralizes the virus.
The mAb is injected in your body and as long as it remains in your body the antibody is there to protect you against the virus.
The controversy around Covid vaccines starts with the creation of spike protein in your body.
The spike protein is how the Covid virus attaches itself to a cell. It is the binding site of the virus.
Source: Invivyd Presentation
The Covid MRNA vaccine instructs your body to create the spike protein. Your body then learns to fight the spike proteins it just made, thereby developing an antibody that can be used when the Covid virus comes along.
The vaccine controversy arises from the concern about creating the foreign spike protein.
Invivyd’s mAb doesn’t compel your body to do anything. It just binds to the spike protein on the Covid virus.
Both the mAb and the vaccine are attacking the Covid virus in the same way – by attaching an antibody to the spike protein on the virus and thereby preventing the virus from binding to cells in your body.
The difference is that the mAb is doing it “passively”. No spike protein is being created, no change at all is being made to your immune system.
Which means vaccine skeptics, like current HHS Secretary Robert Kennedy Jr., have much less to worry about.
That is important. Kennedy is in the process of dismantling the scaffolding of MRNA Covid vaccinations.
On August 27th, the Kennedy’s FDA updated guidance to recommend limiting vaccines to only people over 65, under 6 months old, or that have at least one underlying health condition. That’s a big step down from saying everyone should get a vaccine.
Just this week, the US took it to another level with news they would link Covid shots to the deaths of 25 children.

Source: Washington Post
There is clearly a coordinated effort to undermine existing Covid vaccines. That is really the story here. Moderna (MRNA – NASDAQ) and Pfizer (PFE – NYSE) are having their vaccination ambitions rolled back by the US Food and Drug Administration. What takes their place?
Invivyd is offering a less controversial alternative that does basically the same thing.
THE COVID VACCINES WORK –
BUT THEY ARE FAR FROM PERFECT
It is also one that may actually be more effective – at least now.
The efficacy of vaccines is partly based on a herd response.
If everyone gets a vaccine, the average immunity across the group is high enough that it can wipe out the mobility of the virus.
But the key word there is average. Not everyone responds to vaccination the same way. Some people mount a massive immune response and produce a lot of antibodies. Some people produce very little. I remember reading covid literature in 2020-21 that showed that the response can vary by 100x or even 1,000x between people.
Unfortunately, the people that illicit the smallest response are often those that need the vaccine the most.
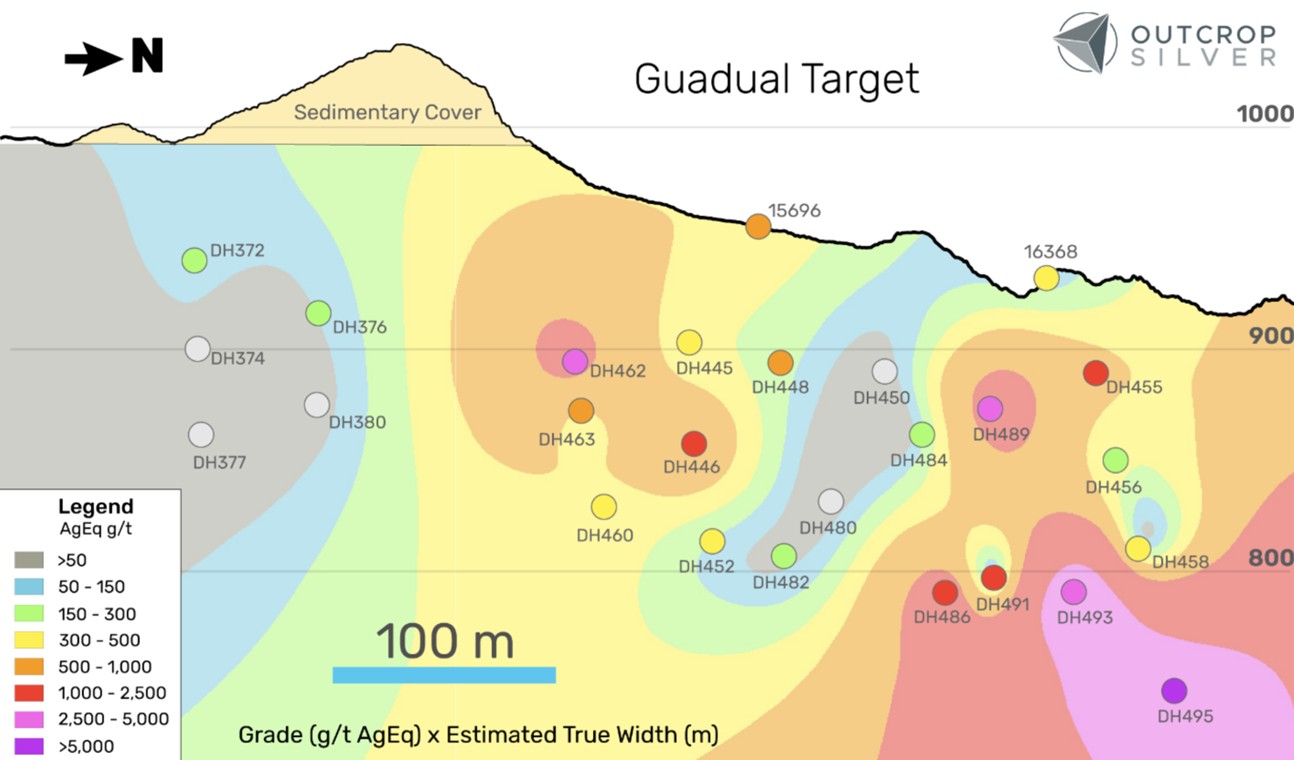
The chart below is not only concerning but indicative of why Invivyd has legs. An immuno-compromised person gets at best a 36% reduced chance of a hospital stay if you were vaccinated in the last 2 months. But this tails off to basically no benefit by 4 months.
Source: Invivyd Investor Presentation
What is happening today, and what is going to get worse under Kennedy, is less of the herd effect. Which means Covid vaccines will be even less effective for the at-risk segments of the population.
People that are at-risk need another option.
FIRST GEN MAB – PEMIVIBART
Invivyd has two Covid-19 drugs, Pemivibart and VYD2311. They also have some earlier stage assets going after measles, RSV and the flu, but those aren’t going to move the needle like the covid drugs will.
Source: Invivyd.com
Their older drug is Pemivibart. In March 2024 Invivyd received an EUA for the use of Pemivibart in the prevention of Covid-19 in adults and kids that have “moderate-to-severe immune compromise that make them unlikely to mount adequate response to vaccination. It is marketed under the name PEMGARDA.
An EUA is a limited use authorization. Invivyd can only sell the drug to people moderately to severely immunocompromised.
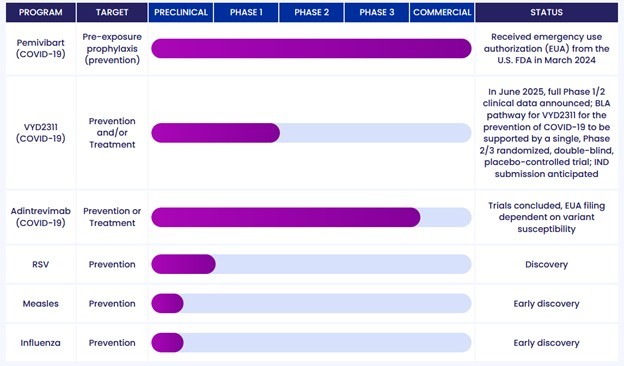
Pemivibart also has its own limitations. It is administered via IV infusion, which means you have to go to a hospital. It also needs to be dosed every 3 months.
All the limitations mean limited revenue. Pemivibart sales are steady, but they are having trouble rising above $11M a quarter.
Source: Invivyd Financials
VYD2311 – DESIGNED WITH VACCINES IN MIND
Invivyd’s next generation mAb, called VYD2311, is designed to get past these limitations and be a true vaccine alternative.
VYD2311 is very similar to Pemivibart. It is 99% structurally the same. Which is good, because Pemivibart works.
VYD2311 was engineered to do everything Pemivibart does but with an experience that mimics existing vaccines.
In a Ph1/2 trial of VYD2311, Invivyd confirmed that the drug works when it is delivered intra-muscularly – that means you can get it with a needle.
That same trial also confirmed that VYD2311 had a half-life of at least 6 months in the body, and that at 6 months the serum levels of the drug in the body remained “high” – higher than Pemivibart.
What the Ph1/2 trial of VYD2311 didn’t address is efficacy – does VYD2311 prevent Covid-19?
For that, Invivyd is are going to need a larger trial.
In August Invivyd announced alignment with the FDA on a rapid pathway to BLA approval. The FDA advised a single Ph2/3 study from a modest number of symptomatic patients could be used for approval.
Most important, Invivyd said that a primary endpoint for the study would be “a reduction in symptomatic COVID-19, resembling CANOPY Cohort B.”
CANOPY is referring to the Ph3 trial in Pemivibart that led to its EUA. Cohort B is one of the two groups of patients that were studied in that trial.
The CANOPY trial looked at two different patient cohorts. Cohort A were immunocompromised people. Cohort B were people that had high exposure to COVID.
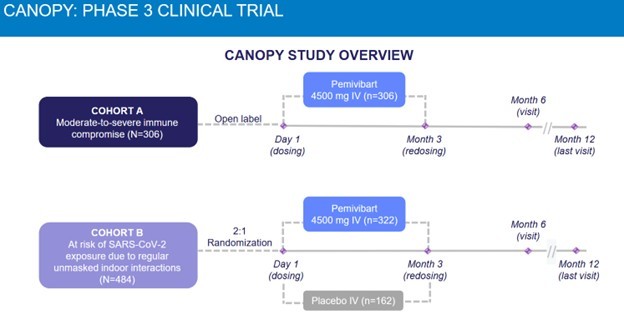
Source: Invivyd CANOPY Phase 3 Presentation
The Cohort B patients were healthy, average people.
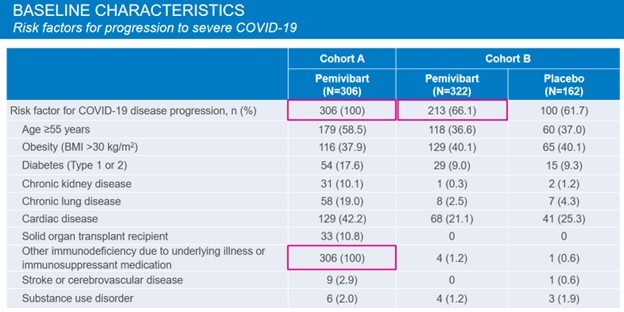
Source: Invivyd CANOPY Phase 3 Presentation
As it turned out, a lot less of Cohort B got Covid-19 if they had a dose of Pemivibart. 19 people out of 160 people in the placebo group got COVID, whereas only 6 out of 317 people in the Pemivibart group got Covid-19. It worked out to an 84.1% reduction in risk.
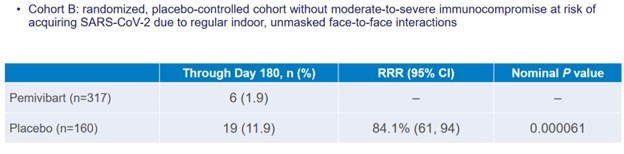
Source: Invivyd Pemivibart Ph3 Results Presentations
Given just how similar VYD2311 is to Pemivibart, it seems reasonable to expect that the drug would work just as well, if not better.
A WHOLE LOT OF UPSIDE
If VYD2311 has a positive Phase 3 trial and is approved for general use, what could that mean for Inviviyd?
In the US in 2024, Covid vaccine uptake was 23% among adults and 14% among children. That works out to 70-80 million people that received a Covid vaccine.
At particular risk are individuals that are immune compromised in some way. This population would include:
1. Older adults (65+), especially those with cardiovascular disease, diabetes, COPD
2. Younger adults with chronic health problems
3. Other groups that have known poor responses to vaccines
Together these groups account for something like 15-20M people in the US.
You can imagine that the overlap between people getting vaccinated today and people at-risk is significant, and with vaccine uptake among the general population so low, they would probably be better served with a mAb, which is going to provide more guaranteed immunity if they come in contact with the virus.
Again, if Invivyd can penetrate 10-20% of the vaccine market, which would assume penetrating most of that at-risk segment, that would be around 7.5-15 million people.
PEMIVIBART is priced at $6,900 per dose. If VYD2311 is really supposed to be a vaccine replacement, it probably has to be priced closer to a vaccine.
Both Moderna (MRNA – NASDAQ) and Pfizer (PFE – NYSE) shots retail at ~$200 per dose.
Another similar mAb is Enflonsia (clesrovimab), a monoclonal antibody for RSV prevention in infants, which is priced at about US $556 per dose.
At a recent Cantor Fitzgerald conference, Chairman of the Board Marc Elia pointed to clesrovimab as a comp, and also said he thought “a turn above” the vaccine pricing would be appropriate.
At $300 per dose for 15 million people, that would be $4.5B of revenue for a single dose per year. If half of that penetrated population receives a second dose every year, that’s another $2.2B.
That puts VYD2311 well above the $1B “blockbuster” status, and we haven’t even considered the opportunity from anywhere other than the US.
Invivyd has a market cap of $300M. They just completed a financing that leaves them with $46M of cash on top of the $34M they had at the end of Q2.
$300M seems like a small price tag for what could be a big payday if VYD2311 can get approved and become a legitimate vaccine alternative.
If the best-case scenario here plays out I have to think the capitalization should grow to multiples of that.
Source: https://oilandgas-investments.com/2025/latest-reports/this-vaccine-alternative-could-be-a-big-winner-invivyd-ivvd-nasd/
Anyone can join.
Anyone can contribute.
Anyone can become informed about their world.
"United We Stand" Click Here To Create Your Personal Citizen Journalist Account Today, Be Sure To Invite Your Friends.
Before It’s News® is a community of individuals who report on what’s going on around them, from all around the world. Anyone can join. Anyone can contribute. Anyone can become informed about their world. "United We Stand" Click Here To Create Your Personal Citizen Journalist Account Today, Be Sure To Invite Your Friends.
LION'S MANE PRODUCT
Try Our Lion’s Mane WHOLE MIND Nootropic Blend 60 Capsules
Mushrooms are having a moment. One fabulous fungus in particular, lion’s mane, may help improve memory, depression and anxiety symptoms. They are also an excellent source of nutrients that show promise as a therapy for dementia, and other neurodegenerative diseases. If you’re living with anxiety or depression, you may be curious about all the therapy options out there — including the natural ones.Our Lion’s Mane WHOLE MIND Nootropic Blend has been formulated to utilize the potency of Lion’s mane but also include the benefits of four other Highly Beneficial Mushrooms. Synergistically, they work together to Build your health through improving cognitive function and immunity regardless of your age. Our Nootropic not only improves your Cognitive Function and Activates your Immune System, but it benefits growth of Essential Gut Flora, further enhancing your Vitality.
Our Formula includes: Lion’s Mane Mushrooms which Increase Brain Power through nerve growth, lessen anxiety, reduce depression, and improve concentration. Its an excellent adaptogen, promotes sleep and improves immunity. Shiitake Mushrooms which Fight cancer cells and infectious disease, boost the immune system, promotes brain function, and serves as a source of B vitamins. Maitake Mushrooms which regulate blood sugar levels of diabetics, reduce hypertension and boosts the immune system. Reishi Mushrooms which Fight inflammation, liver disease, fatigue, tumor growth and cancer. They Improve skin disorders and soothes digestive problems, stomach ulcers and leaky gut syndrome. Chaga Mushrooms which have anti-aging effects, boost immune function, improve stamina and athletic performance, even act as a natural aphrodisiac, fighting diabetes and improving liver function. Try Our Lion’s Mane WHOLE MIND Nootropic Blend 60 Capsules Today. Be 100% Satisfied or Receive a Full Money Back Guarantee. Order Yours Today by Following This Link.






