So much so for “Radical Transparency” in food safety
According to the HHS website under the notion of “Radical Transparency:”
“HHS and FDA are committed to promoting radical transparency to make sure all Americans know what is in their food.”

I guess that is just not always? Thanks to Food Safety News for covering this.
The Food and Drug Administration has closed investigations into two Salmonella outbreaks and released redacted information about them.
The information, officially referred to as Executive Incident Summary Abstracts, is part of Health and Human Services Secretary Robert F. Kennedy’s plan to increase transparency. However, the reports on the two outbreaks do not name restaurants or stores or growers associated with the outbreaks.
For an outbreak of Salmonella Oanienburg traced to pistachio cream, the FDA has filed an Executive Incident Summary Abstract that includes previously unreleased information. The abstract reports that the outbreak was traced to a restaurant but the name of the restaurant has been redacted from the report. The outbreak sickened four people.

“On 6/10/2025, OII OHFI Central 3, notified CORE (Coordinated Outbreak Response & Evaluation (CORE) Network) Signals about a cluster of Salmonella Oranienburg illnesses associated with a single restaurant exposure in (redacted) Minnesota,” according to the abstract. “All three cases in Minnesota and one case in New Jersey reported consuming similar food items that included pistachio cream, which led to Minnesota health officials to collect and test product. Samples tested in Minnesota matched the outbreak strain by Whole Genome Sequencing (WGS).”
In its abstract the FDA reports that response activities were initiated by the identification of Salmonella Oranienburg from state samples of sealed and unsealed pistachio cream collected from a single point of service, but the agency did not name that point of service. The three Minnesota patients all consumed Emek brand pistachio cream imported from Turkey and served on crepes at a single restaurant location, but again, the FDA did not name the restaurant.
There was one routine FDA import sample collected as well as samples collected via COREs import bulletin (redacted) from (redacted) importers, according to the FDA abstract.
The FDA also reported that New Jersey officials collected retail samples from multiple retail stores — which the FDA did not name in its abstract — mentioned by the New Jersey patient; all NJ samples tested negative for Salmonella. Retail samples of Emek Spread Pistachio Cacao Cream with Kadayif were also collected from World Market stores by state partners in Minnesota and Maryland.
Whole Genome Sequence (WGS) was conducted on all positive samples collected at import and at World Market and a total of 28 positive samples were identified to belong to 6 different serovars/strains including: Salmonella Oranienburg, Salmonella Reading, Salmonella Liverpool, Salmonella Meleagridis, Salmonella Mbandaka and Salmonella Montevideo.
Emek Dogal Saglik Urunleri Iklimlendirme Gida Insaat San Ve Tic Ltd Sti was added to Import Alert 99-43 as of Sept. 23, meaning its product can no longe be imposed to the United States.
An investigation into another outbreak of Salmonella Oranienburg has been closed and described in a heavily redacted abstract. The outbreak sickened 25 people but the FDA has not reported the patients’ ages or where they live.
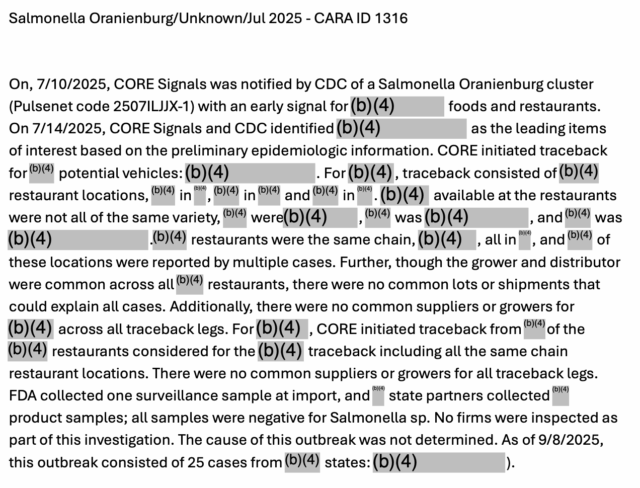
“On, 7/10/2025, CORE Signals was notified by CDC of a Salmonella Oranienburg cluster (Pulsenet code 2507ILJJX-1) with an early signal for (redacted) foods and restaurants. On 7/14/2025, CORE Signals and CDC identified (redacted) as the leading items of interest based on the preliminary epidemiologic information,” according to the abstract.”
“CORE initiated traceback or potential vehicles: For (redacted), traceback consisted of (redacted) restaurant locations, in (redacted), in (redacted) and in (redacted).
“(Redacted) available at the restaurants were not all of the same variety, were (redacted), (redacted) was, (redacted) and was (redacted). (Redacted) restaurants were the same chain, all in (redacted), and of (redacted) these locations were reported by multiple cases.
“Further, though the grower and distributor were common across all (redacted) restaurants, there were no common lots or shipments that could explain all cases. Additionally, there were no common suppliers or growers for (redacted) (redacted)(redacted) across all traceback legs.
“For (redacted), CORE initiated traceback from of the (redacted) (redacted)restaurants considered for the traceback including all the same chain restaurant locations. There were no common suppliers or growers for all traceback legs.
“(Redacted) (redacted) FDA collected one surveillance sample at import, and state partners collected product samples; all samples were negative for Salmonella sp.
“No firms were inspected as part of this investigation. The cause of this outbreak was not determined. As of 9/8/2025, (b)(4) this outbreak consisted of 25 cases from states (redacted).”
Republished with permission from Bill Marler and Marler Clark. Copyright (c) Marler Clark LLP, PS. All rights reserved.
Source: https://www.marlerblog.com/case-news/so-much-so-for-radical-transparency-in-food-safety/
Anyone can join.
Anyone can contribute.
Anyone can become informed about their world.
"United We Stand" Click Here To Create Your Personal Citizen Journalist Account Today, Be Sure To Invite Your Friends.
Before It’s News® is a community of individuals who report on what’s going on around them, from all around the world. Anyone can join. Anyone can contribute. Anyone can become informed about their world. "United We Stand" Click Here To Create Your Personal Citizen Journalist Account Today, Be Sure To Invite Your Friends.
LION'S MANE PRODUCT
Try Our Lion’s Mane WHOLE MIND Nootropic Blend 60 Capsules
Mushrooms are having a moment. One fabulous fungus in particular, lion’s mane, may help improve memory, depression and anxiety symptoms. They are also an excellent source of nutrients that show promise as a therapy for dementia, and other neurodegenerative diseases. If you’re living with anxiety or depression, you may be curious about all the therapy options out there — including the natural ones.Our Lion’s Mane WHOLE MIND Nootropic Blend has been formulated to utilize the potency of Lion’s mane but also include the benefits of four other Highly Beneficial Mushrooms. Synergistically, they work together to Build your health through improving cognitive function and immunity regardless of your age. Our Nootropic not only improves your Cognitive Function and Activates your Immune System, but it benefits growth of Essential Gut Flora, further enhancing your Vitality.
Our Formula includes: Lion’s Mane Mushrooms which Increase Brain Power through nerve growth, lessen anxiety, reduce depression, and improve concentration. Its an excellent adaptogen, promotes sleep and improves immunity. Shiitake Mushrooms which Fight cancer cells and infectious disease, boost the immune system, promotes brain function, and serves as a source of B vitamins. Maitake Mushrooms which regulate blood sugar levels of diabetics, reduce hypertension and boosts the immune system. Reishi Mushrooms which Fight inflammation, liver disease, fatigue, tumor growth and cancer. They Improve skin disorders and soothes digestive problems, stomach ulcers and leaky gut syndrome. Chaga Mushrooms which have anti-aging effects, boost immune function, improve stamina and athletic performance, even act as a natural aphrodisiac, fighting diabetes and improving liver function. Try Our Lion’s Mane WHOLE MIND Nootropic Blend 60 Capsules Today. Be 100% Satisfied or Receive a Full Money Back Guarantee. Order Yours Today by Following This Link.






