Is ByHeart Formula still on Store Shelves? Have kids become infected with Botulism AFTER the Recall?
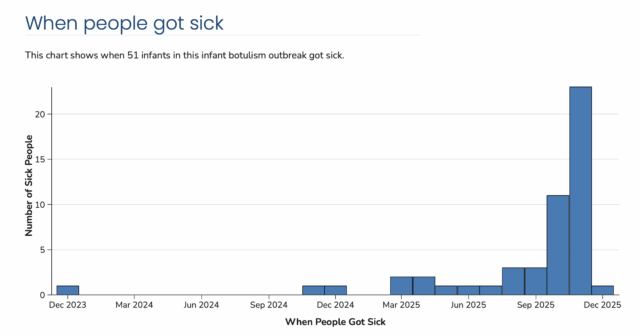
Here are some facts. Incubation period for Infant Botulism is 3-30 days. The first Recall was November 8th when only 13 children were reported ill. As late as December 3rd (possibly later) they (FDA and State Public Health) were still finding cans on shelves. Any illnesses after Mid-November could well be coming from product that should have been recalled but was still purchased and consumed. And there still might be more reported illnesses in the coming weeks. Besides stores, what about places like food banks? Or all the overseas sales? Or in the back of families pantries?
Seriously, S I said the other day, babies getting botulism and stores cannot seem to get the poison off shelves?
If any store sold this product AFTER the recall date, and a kid became ill, they are subject to punitive damages.
Here are some excerpts from FDA Warning Letters of a few days ago. Links below are the full letters.
Target
On November 19, 2025, FDA held a call with you to discuss the ineffectiveness of the recall within your Target stores. During this discussion, FDA requested information regarding actions you were prepared to implement to ensure recalled product was no longer available for purchase at Target stores nationwide. Despite follow-up emails from the FDA on November 20, 21, 24, and 26, 2025 and December 1, 3, and 8, 2025, you have not provided FDA with any information demonstrating that corrective actions to effectuate this recall have been implemented throughout your organization to prevent adulterated food from being received in interstate commerce and subsequently offered for sale.
The inadequacy of Target’s recall response was further demonstrated on November 20, 2025, when Arkansas state partners observed ByHeart Whole Nutrition Infant Formula single-serve “anywhere pack” sticks on a Target store shelf with promotional “Sale!” signage offering a $2.00 discount on the recalled formula from November 16 to November 22, 2025. This observation indicates not only Target’s failure to remove recalled infant formula from the store shelves, but the active promotion and discounted sale of recalled infant formula product implicated in an infant botulism outbreak, more than a (b)(4) after Target was first made aware of ByHeart’s expanded recall.
Walmart
However, based on FDA’s review of information from state and local partners, the Agency determined that recalled ByHeart Whole Nutrition Infant Formula remained on shelves at (b)(4) Walmart store locations across 21 states from November 12 to November 26, 2025. This represents a period of (b)(4) days after Walmart was notified of the recall expansion. State and local partners reported several explanations offered by Walmart store associates for the continued presence of the recalled product, including lack of awareness of the recall notice, confusion regarding which specific lots were affected, failure to remove all impacted product, and stocking products that arrived after the recall notification.
On November 18, 2025, FDA held a call with you to discuss the ineffectiveness of the recall within your Walmart stores. During this discussion, FDA requested information regarding actions you were prepared to implement to ensure recalled product was no longer available for purchase at Walmart stores nationwide. Despite follow-up emails from the FDA on November 20, 21, and 24, 2025, and December 1, 3, and 8, 2025, you have not provided FDA with any information demonstrating that corrective actions to effectuate this recall have been implemented throughout your organization to prevent adulterated food from being received in interstate commerce and subsequently offered for sale.
Kroger
However, based on FDA’s review of information from state and local partners, the Agency determined that recalled ByHeart Whole Nutrition Infant Formula remained on shelves at (b)(4) Kroger store locations across 10 states from November 12, 2025, to November 19, 2025. This represents a period of (b)(4) days after Kroger was first notified of the initial recall and (b)(4) days after Kroger was notified of the recall expansion. State and local partners reported several explanations offered by Kroger store associates for the continued presence of the recalled product, including lack of awareness of the recall notice, confusion regarding which specific lots were affected, failure to remove all impacted product, and stocking products that arrived after the recall notification.
On November 19, 2025, FDA held a call with you to discuss the ineffectiveness of the recall within your Kroger stores. During this discussion, FDA requested information regarding actions you were prepared to implement to ensure recalled product was no longer available for purchase at Kroger stores nationwide. Despite follow-up emails from the FDA on November 20, 21, 24, and 25, 2025 and December 1, 3, and 8, 2025, you have not provided FDA with any information demonstrating that corrective actions to effectuate this recall have been implemented throughout your organization to prevent adulterated food from being received in interstate commerce and subsequently offered for sale.
Albertsons
However, based on FDA’s review of information from state and local partners, the Agency determined that recalled ByHeart Whole Nutrition Infant Formula remained on shelves at (b)(4) Albertsons store locations across 11 states from November 12 to November 19, 2025. This represents a period of (b)(4) days after Albertsons was first notified of the initial recall and (b)(4) days after Albertsons was notified of the recall expansion. State and local partners reported several explanations offered by Albertsons store associates for the continued presence of the recalled product, including lack of awareness of the recall notice, confusion regarding which specific lots were affected, failure to remove all impacted product, and stocking products that arrived after the recall notification.
On November 20, 2025, FDA held a call with you to discuss the ineffectiveness of the recall within your Albertsons stores. During this discussion, FDA requested information regarding actions you were prepared to implement to ensure recalled product was no longer available for purchase at Albertsons stores nationwide. Despite follow-up emails from the FDA on November 20 and 24, 2025, and December 1 and 8, 2025, you have not provided FDA with any information demonstrating that corrective actions to effectuate this recall have been implemented throughout your organization to prevent adulterated food from being received in interstate commerce and subsequently offered for sale.
We need to do better.
Republished with permission from Bill Marler and Marler Clark. Copyright (c) Marler Clark LLP, PS. All rights reserved.
Source: https://www.marlerblog.com/case-news/is-byheart-formula-still-on-store-shelves-have-kids-become-infected-with-botulism-after-the-recall/
Anyone can join.
Anyone can contribute.
Anyone can become informed about their world.
"United We Stand" Click Here To Create Your Personal Citizen Journalist Account Today, Be Sure To Invite Your Friends.
Before It’s News® is a community of individuals who report on what’s going on around them, from all around the world. Anyone can join. Anyone can contribute. Anyone can become informed about their world. "United We Stand" Click Here To Create Your Personal Citizen Journalist Account Today, Be Sure To Invite Your Friends.
LION'S MANE PRODUCT
Try Our Lion’s Mane WHOLE MIND Nootropic Blend 60 Capsules
Mushrooms are having a moment. One fabulous fungus in particular, lion’s mane, may help improve memory, depression and anxiety symptoms. They are also an excellent source of nutrients that show promise as a therapy for dementia, and other neurodegenerative diseases. If you’re living with anxiety or depression, you may be curious about all the therapy options out there — including the natural ones.Our Lion’s Mane WHOLE MIND Nootropic Blend has been formulated to utilize the potency of Lion’s mane but also include the benefits of four other Highly Beneficial Mushrooms. Synergistically, they work together to Build your health through improving cognitive function and immunity regardless of your age. Our Nootropic not only improves your Cognitive Function and Activates your Immune System, but it benefits growth of Essential Gut Flora, further enhancing your Vitality.
Our Formula includes: Lion’s Mane Mushrooms which Increase Brain Power through nerve growth, lessen anxiety, reduce depression, and improve concentration. Its an excellent adaptogen, promotes sleep and improves immunity. Shiitake Mushrooms which Fight cancer cells and infectious disease, boost the immune system, promotes brain function, and serves as a source of B vitamins. Maitake Mushrooms which regulate blood sugar levels of diabetics, reduce hypertension and boosts the immune system. Reishi Mushrooms which Fight inflammation, liver disease, fatigue, tumor growth and cancer. They Improve skin disorders and soothes digestive problems, stomach ulcers and leaky gut syndrome. Chaga Mushrooms which have anti-aging effects, boost immune function, improve stamina and athletic performance, even act as a natural aphrodisiac, fighting diabetes and improving liver function. Try Our Lion’s Mane WHOLE MIND Nootropic Blend 60 Capsules Today. Be 100% Satisfied or Receive a Full Money Back Guarantee. Order Yours Today by Following This Link.






