What caused the ByHeart Botulism Outbreak that went on for years?
Contaminated supplies? Contaminated plaint? Lots of questions.
According to recent reports, the FDA cannot rule out the possibility that contamination might have affected all ByHeart formula products. In response, CDC broadened the case definition to include any infant with botulism who was exposed to ByHeart formula at any time since the product’s release in March 2022.
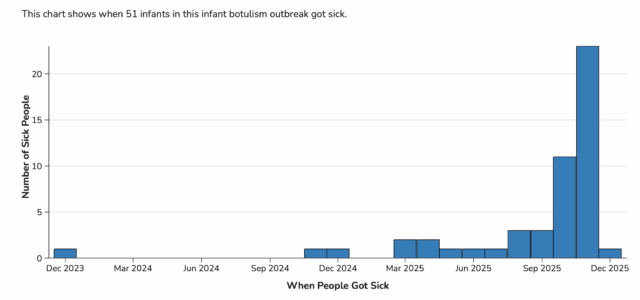
As of December 10, 2025, a total of 51 infants with suspected or confirmed infant botulism and confirmed exposure to ByHeart Whole Nutrition infant formula have been reported from 19 states.
Previously, case counts included illnesses from August 1, 2025, onward. With the expanded definition, CDC and state partners identified 10 additional prior cases that occurred from December 2023 through July 2025. At this time, no cases have been identified between March 2022 and December 2023. All 10 prior cases are confirmed infant botulism cases with documented exposure to ByHeart formula.
Laboratory confirmation for some cases is ongoing. Illnesses started on dates ranging from December 24, 2023 to December 1, 2025. All 51 infants were hospitalized.
The ByHeart infant formula recall impacts markets outside the United States. Customer information provided by Amazon shows that a limited quantity of recalled ByHeart infant formula was distributed to Argentina, Brazil, Brunei, Canada, Chile, China, Colombia, Ecuador, Egypt, Hong Kong, Israel, Jamaica, Japan, Republic of Korea, Peru, Philippines, Romania, Singapore, South Africa, Thailand, and the British Virgin Islands.
ByHeart, Inc. is the parent company for three manufacturing / packaging facilities:
- Blendhouse LLC (Reading, PA), a manufacturing site. Closed in 2024.
- Blendhouse Allerton, LLC (Allerton, IA), a manufacturing site.
- Blendhouse Portland LLC (Portland, OR), a packaging site.
Of these, the Reading facility manufactures the infant formula base product, which is then blended and packaged at a different facility. The Reading location achieved its FDA registration on April 28, 2022 and was subjected to an initial, and successful, FDA inspection in June 2022. When child illnesses were linked to Cronobacter sakazakii and infant formula in 2022, the FDA chose to take an in-depth look at all the powdered infant formula manufacturing sites, including ByHeart’s Reading facility. What they found was disturbing, resulting in both inspections being classified as “Official Action Indicated.”
The FDA investigation team uncovered numerous problems, which were summarized in a Warning Letter, dated August 30, 2023. These included:
- Lack of process control system, as evidenced by a finding of Cronobacter sakazakii in a batch of ByHeart Whole Nutrition Infant Formula finished product. The infant formula base which was incorporated into that batch had been manufactured in continuous process from July 13, 2022 through August 23, 2022.
- Discrepancy between company’s root cause analysis of the Cronobacter contamination problem and the conclusion of the third-party lab, in which the company blamed lab error and the lab denied that they had erred.
- Multiple notifications from third party lab of positive Cronobacter sakazakii findings from July 25, 2022 through August 27, 2022 within the processing environment.
- Two water events, during which water leaked into the manufacturing areas from outside.
The FDA conducted its next inspection eleven months later. According to information posted on the FDA’s inspection data dashboard, investigators uncovered several serious problems. ByHeart:
- did not implement a system of production and in-process controls for an infant formula
- did not maintain a building used in the manufacture, processing, packing or holding of infant formula in a clean and sanitary condition
- did not minimize the potential for contamination of raw materials using appropriate measures
- did not ensure that all surfaces that contacted ingredients, in-process materials and infant formula were cleaned and sanitized and maintained to protect infant formula from being contaminated by any source
- did not monitor the temperature in a thermal processing equipment at a point where temperature control is necessary to prevent adulteration
- did not exclude pests from your food plant to protect against contamination of food
See Warning Letter 2023, Inspection Report 2022, 483 2023 and Inspection Report 2024.
The FDA inspected the Blendhouse Allerton facility in 2025. Its Inspection Report stated:
At the close-out of the inspection, a Form FDA 483, Inspectional Observations, was issued for 3 items, along with 2 “Additional Observations”, and 7 “General Discussion with Management”. The three 483 items included, receiving, and releasing [redacted] ingredient used in infant formula base powder that was not held under conditions to prevent adulteration, not taking actions to eliminate all potential harborage areas when issues with rodent arose during the year 2024-2025; and not monitoring the floor conditions adequately at the dryer [redacted] (level [redacted] and level [redacted]) when there were findings of confirmed Cronobacter Sakazakii. The two additional observations consists of the firm not having clear barriers separating hygiene zones; and not monitoring bathhouse differential pressures.
Also shared with the firm on February 14, 2025, was that the FDA observed “consecutive [redacted] pest control service tickets between October 2024 and December 2024 that reported up to 200 large black flies caught in insect light traps in the IF [redacted] .”
Further, Form 483 2025 stated:
“Observation 1: You approved and released for use an ingredient that was not manufactured, packaged, labeled, or held under conditions to prevent adulteration.” “Observation 2: You did not exclude pests from your food plant to protect against contamination of food.” “Observation 3: You did not maintain a building used in the manufacture, processing, packing or holding of infant formula in a clean and sanitary condition.”
The FDA also inspected the Blendhouse Portland facility in 2025. Its Inspection Report found consumer complaints of infant illnesses: Salmonella – 8 Complaints, Campylobacter – 1 Complaint 1, E. coli – 1 Complaint.
Contaminated supplies? Contaminated plaint? Lots of questions.
Republished with permission from Bill Marler and Marler Clark. Copyright (c) Marler Clark LLP, PS. All rights reserved.
Source: https://www.marlerblog.com/case-news/what-caused-the-byheart-botulism-outbreak-that-went-on-for-years/
Anyone can join.
Anyone can contribute.
Anyone can become informed about their world.
"United We Stand" Click Here To Create Your Personal Citizen Journalist Account Today, Be Sure To Invite Your Friends.
Before It’s News® is a community of individuals who report on what’s going on around them, from all around the world. Anyone can join. Anyone can contribute. Anyone can become informed about their world. "United We Stand" Click Here To Create Your Personal Citizen Journalist Account Today, Be Sure To Invite Your Friends.
LION'S MANE PRODUCT
Try Our Lion’s Mane WHOLE MIND Nootropic Blend 60 Capsules
Mushrooms are having a moment. One fabulous fungus in particular, lion’s mane, may help improve memory, depression and anxiety symptoms. They are also an excellent source of nutrients that show promise as a therapy for dementia, and other neurodegenerative diseases. If you’re living with anxiety or depression, you may be curious about all the therapy options out there — including the natural ones.Our Lion’s Mane WHOLE MIND Nootropic Blend has been formulated to utilize the potency of Lion’s mane but also include the benefits of four other Highly Beneficial Mushrooms. Synergistically, they work together to Build your health through improving cognitive function and immunity regardless of your age. Our Nootropic not only improves your Cognitive Function and Activates your Immune System, but it benefits growth of Essential Gut Flora, further enhancing your Vitality.
Our Formula includes: Lion’s Mane Mushrooms which Increase Brain Power through nerve growth, lessen anxiety, reduce depression, and improve concentration. Its an excellent adaptogen, promotes sleep and improves immunity. Shiitake Mushrooms which Fight cancer cells and infectious disease, boost the immune system, promotes brain function, and serves as a source of B vitamins. Maitake Mushrooms which regulate blood sugar levels of diabetics, reduce hypertension and boosts the immune system. Reishi Mushrooms which Fight inflammation, liver disease, fatigue, tumor growth and cancer. They Improve skin disorders and soothes digestive problems, stomach ulcers and leaky gut syndrome. Chaga Mushrooms which have anti-aging effects, boost immune function, improve stamina and athletic performance, even act as a natural aphrodisiac, fighting diabetes and improving liver function. Try Our Lion’s Mane WHOLE MIND Nootropic Blend 60 Capsules Today. Be 100% Satisfied or Receive a Full Money Back Guarantee. Order Yours Today by Following This Link.






